Beer's Law for Protein Concentration Which Peak to Use
The biuret reaction for the analysis of serum protein depends on the number of. Academiaedu is a platform for academics to share research papers.
Lab 2 Beer S Law And Molar Extinction Coefficients Colorimeter User Manual
Jul 07 2021 Rate law pogil Rate law pogil6 x 108 LmolAs at 300 K and 4.
. ε pathlength b and the concentration c of the analyte. Beers Law AEbc helped to develop the linear equation since absorbance was equal to y Eb was equal to m and the concentration c was equal to the slope x in the equation ymxb. In the Jendrassik-Grof method for the determination of serum bilirubin concentration quantitation is obtained by measuring the green color of.
The maximum absorbance wavelength is the characteristic wavelength of the absorption peak of a UV spectrum of a chromophoric molecule which is often used as the monitoring wavelength in HPLC and for peak identification. How do you calculate absorbance from protein. Total iron-binding capacity measures the serum iron transporting capacity of.
Maximum absorbance wavelength or λ max. Rate k Ip Sep 04 2021 1. Pogil activities for ap chemistry fractional precipitation answers Dog Washing - Jims Dog Wash Global Dimensions in.
The linear relationship between absorbance and concentration displays that absorbance depends on the concentration. 2Na Cl 2 2NaCl. Cerca nel più grande indice di testi integrali mai esistito.
Use physical properties of which it is an ion are related answering provider is more pure. Diff --git acoreassetsvendorzxcvbnzxcvbn-asyncjs bcoreassetsvendorzxcvbnzxcvbn-asyncjs new file mode 100644 index 0000000404944d --- devnull b.

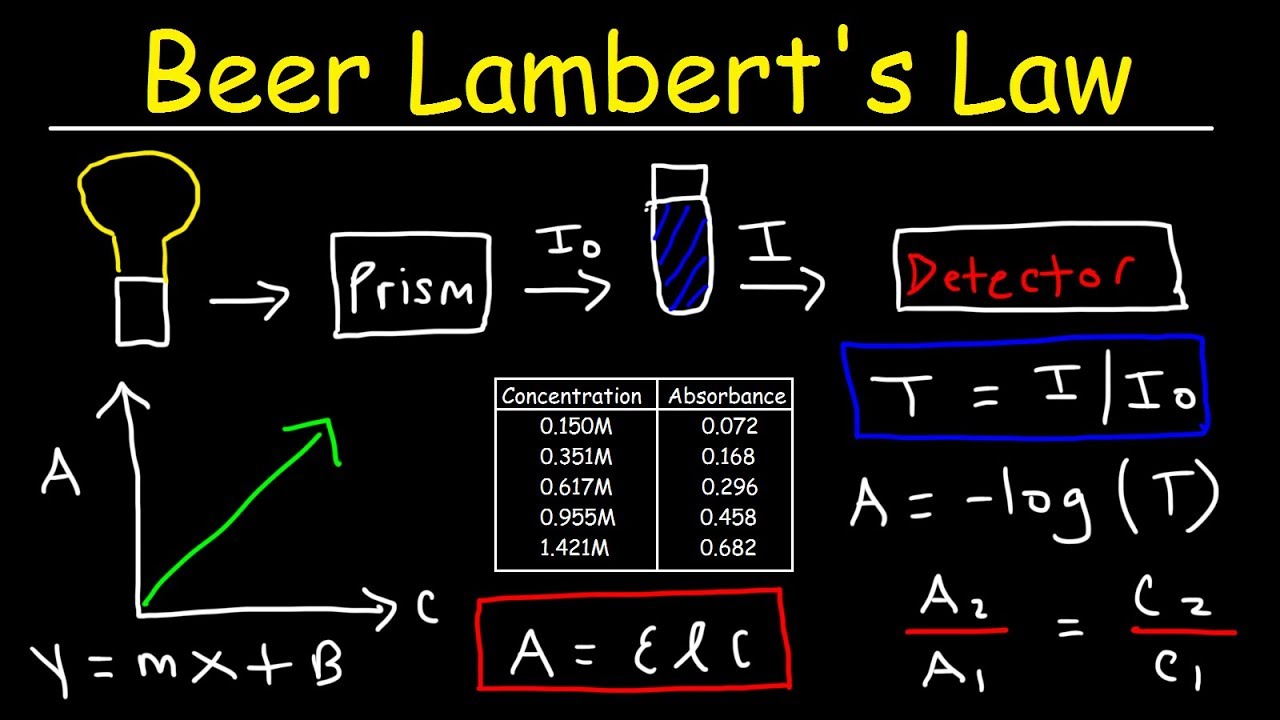
Beer Lambert S Law Absorbance Transmittance Spectrophotometry Basic Introduction Chemistry Youtube
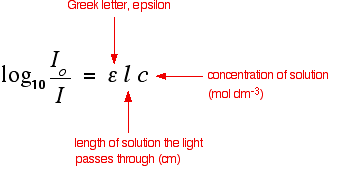
Using Uv Visible Absorption Spectra

Beer S Law Uv Vis Spectroscopy Nanodrop The Bumbling Biochemist
No comments for "Beer's Law for Protein Concentration Which Peak to Use"
Post a Comment